Judge Says TX Hospital's Requirement Making Employees Take COVID Shot or Get Fired is ‘Not Coercion but a Choice.’ Despite the Lack of FDA Approval Judge says the "Vaccine" is "Not Experimental"
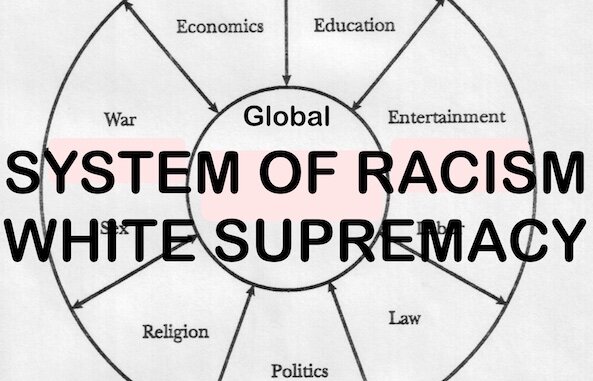
/ROBERT HIGGS EXPLAINS, “DIRECTING FEAR IN A SOCIETY IS TANTAMOUNT TO CONTROLLING THAT SOCIETY.” LARKEN ROSE STATES, “ALMOST ALL OPPRESSION VIA PROPAGANDA IS BASED UPON SCARING PEOPLE, AND THEN PRESENTING A FALSE CHOICE WHERE THE PEOPLE CAN CHOOSE EITHER TO DO WHAT YOU WANT THEM TO DO, OR FACE SOME UNKNOWN (OFTEN PURELY FICTIONAL) HORROR.” LYSANDER SPOONER EXPLAINS THAT ALL GOVERNMENTS RULE THROUGH AND CREATE LAWS THROUGH SOME FORM OF “EMERGENCY” OR CONSPIRACY.
From [HERE] and [HERE] A Texas hospital system’s mandatory COVID-19 vaccination policy for employees can stand after a federal judge on Saturday dismissed a closely watched lawsuit from workers refusing to get the shot.
Southern District of Texas Judge Lynn Hughes made his decision days after Houston Methodist Hospital suspended 178 workers for not getting vaccinated by a June 7 deadline.
The court’s pre-trial ruling was made without the benefit of receiving testimony or other actual evidence.
The hospital system’s policies were not coercion against staff, Hughes said. They were a choice the hospital system made “to keep staff, patients, and their families safer.”
The 117 suing workers, including plaintiff Jennifer Bridges, a nurse for almost seven years at the hospital system, had their own choices to make, the judge said. Bridges and other plaintiffs had every right to accept or refuse the vaccine. “If she refuses, she will simply need to work somewhere else,” the decision said.
Hughes wrote that employers could impose consequences for noncompliance on all sorts of rules, far beyond vaccination.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired. Every employment includes limits on the worker’s behavior in exchange for his remuneration. That is all part of the bargain.”
But Jared Woodfill, the lawyer for the suing workers, vowed to appeal the case all the way up to the Supreme Court. “This is just one battle in a larger war to protect the rights of employees … All of my clients continue to be committed to fighting this unjust policy.” [MORE]
The complaint states “None of the currently available experimental vaccines for COVID-19 has received final approval from the FDA. Rather, each one of the COVID-19 experimental vaccines is an unapproved product that has been granted EAU. The FDA refers to the COVID-19 experimental vaccine as “investigational products”, meaning they remain classified as experimental.“ It further explains:
It is undisputed that the vaccine being forced upon Plaintiffs is “unapproved”. Even though the FDA granted emergency use authorization for the Pfizer/BioNTech and Moderna vaccines in December 2020, the clinical trials the FDA will rely upon to ultimately decide whether to license these and other COVID-19 experimental vaccines are still underway and are designed to last for approximately two (2) years to collect adequate data to establish if these vaccines are safe and effective enough for the FDA to approve. The abbreviated timelines for the emergency use applications and authorizations means there is much the FDA does not know about these products even as it authorizes them for emergency use, including their effectiveness against infection, death, and transmission of SARS-CoV-2, the virus that is allegedly the cause of the COVID disease. Given the uncertainty about the COVID-19 experimental vaccines, the FDA requires that each dose of the experimental vaccine shall have a label that states that the product is an emergency use authorization, that the EAU is explicit that each is “an investigational vaccine not licensed for any indication” and that all “promotional material relating to the Covid-19 Vaccine clearly and conspicuously…state that this product has not been approved or licensed by the FDA, but has been authorized for emergency use by FDA”.
The complaint also explains that the COVID “vaccine” has caused an extraordinarily high number of reports of illness to be reported in the VAERS system which under normal circumstances would cause it from being pulled off the market. It states;
In 1990, the Vaccine Adverse Event Reporting Systems (“VAERS”) was established as a national early warning system to detect possible safety problems in U.S. licensed vaccines. VAERS is a passive reporting system, meaning it relies on individuals to voluntarily send in reports of their experiences to CDC and FDA. VAERS is useful in detecting unusual or unexpected patterns of adverse event reporting that might indicate a possible safety problem with a vaccine. This way, VAERS can provide CDC and FDA with valuable information that additional work and evaluation is necessary to further assess a possible safety concern.
There were 4,434 death reports and over 12,619 serious injuries reported to the CDC's VAERS database from COVID-19 vaccines through May 10, 2021. By comparison, from July 1, 1997, until December 31, 2013, VAERS received 666 adult death reports.3 The flu vaccines are linked to 20–30 death reports a year, according to Dr. Peter McCullough, and those 20–30 death reports come with considerably more vaccines administered. Arguably, if the experimental vaccine was any other vaccine or drug, it would already have been removed from the market. Usually, a new drug is withdrawn after 50 deaths, which is not typical because the FDA has a strict approval process. The COVID-19 vaccines have been exempted from the approval process, instead being temporarily "authorized" for emergency use.
Thirty-five hundred plus (3,500 +) reports is 70 times the normal threshold for pulling a drug from the market. Although this is raw data, previous VAERS studies have shown that only 1-10% of vaccine-related deaths are reported to VAERS —or less. The COVID vaccines are adding a year's worth of VAERS reports every week. In just four months, more adverse reports were added to the VAERS database than any single vaccine has had cumulatively over the past 31 years. This is clearly a safety signal, further studies need to be done and Plaintiffs should not be forced to participate in these dangerous trials as a condition for employment. [MORE]