DC Forces Genocidal COVID Shots on Kids Ages 12+. Law Destroys Rights to Refuse Medical Treatment and Refuse Emergency Use Vaccines. Falsely Claims Vax Slows Spread, Disproportionately Affects Blacks
/COVID Injections Don't Immunize or Stop Spread. They're Treatments (Not Vaccines) that feed the Virus and Facilitate its Development into More Variants. The Government IS TRYING TO KILL YOU.
From [HERE] and [HERE] D.C. schools have a new mandate requiring all students 12 and up to receive the experimental COVID-19 vaccine before returning to school this fall.
In a July 19 press release, the Office of the State Superintendent of Education directed that all students eligible for COVID-19 vaccines according to the Food and Drug Administration (FDA) must receive their vaccines for the 2022-2023 school year.
The mandate extends to all schools, including private, parochial, and independent. Students must verify their vaccine status as part of enrollment and attendance requirements.
According to the mandate, all students 12 and up, unless exempted, must have received a series of COVID-19 shots or begun the process of vaccination before the start of the school year. Pursuant to implementing regulations, “School authorities may exclude from regular instruction a student who is not immunized and provide for special instruction for the student.” DCMR 5- 5300.13
With regard to exemptions the new law states, § 38–506. Exemption from certification.
“No certification of immunization shall be required for the admission to a school of a student:
(1) For whom the responsible person objects in good faith and in writing, to the chief official of the school, that immunization would violate his or her religious beliefs; or
(2) For whom the school has written certification by a private physician, his or her representative, or the public health authorities that immunization is medically inadvisable.” D.C. Code § 38–506.
It makes no exception for children with natural immunity.
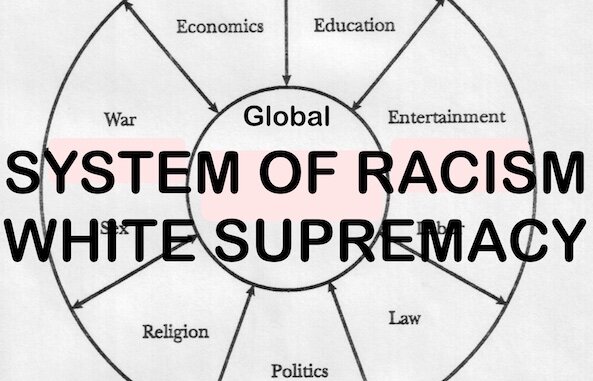
The legislative record indicates that DC lawmakers were explicitly warned that COVID injections are dangerous to all people. The liberal puppeticians were also aware that the mandate will disproportionately impact Black people. The record states, “In a school system made up of mainly Black students, this Council is determined to override the decision‐making of Black parents about the medical care of their own children. This is pure white supremacy.“ [MORE]
The mandate is part of the implementation of a COVID-19 vaccination law passed in D.C. last year. The law required all eligible teachers and students to receive the experimental vaccines.
NO FULLY APPROVED COVID SHOTS ARE AVAILABLE IN THE US. Despite evidence showing the vaccine is more dangerous to children than the virus on July 7th the U.S. Food and Drug Administration (FDA) granted full approval of Pfizer-BioNTech’s Comirnaty COVID-19 vaccine for adolescents 12 through 15 years old.Similarly, the Centers for Disease Control Prevention (CDC) recently approved the experimental shots for children as young as six months old.
However, Comirnaty is not available in the U.S for any age group and is not the same formula as the Pfizer-BioNTech vaccine currently authorized under EUA and being distributed as a “fully approved” vaccine.
That is, all all the shots are Emergency Use, which are legally distinct, not interchangeable w/comirnaty. As such, the only way to comply with the D.C.’s mandate will be children are injected with a shot under an EUA. This is especially important because EUA vaccines bypass the FDA and PHS Act's requirements for safety and efficacy.
Pfizer’s information hotline says it has no specific information on when Comirnaty will be available. The FDA said earlier this month that the Pfizer-BioNTech vaccine “has been, and will continue to be, authorized for emergency use in this age group since May 2021.” The CDC’s website states that Comirnaty is “not orderable.”
According to FDA documents, Comirnaty is not available in the U.S. and nobody has received a fully approved and licensed COVID-19 vaccine.
“Comirnaty has not been made available under EUA,” said Dr. Madhava Setty, physician and senior science editor for The Defender. “The FDA and Pfizer have already stated very quietly, that they have no intent of manufacturing Comirnaty for distribution. Everyone is getting the non-licensed formulation that carries no liability for pharmaceutical companies.” [MORE]
Apparently in the recent case NFIB v. OSHA before the US Supreme Court, it was undisputed by the government that “Comirnaty is not available at all in the United States.”
The distinction between an EUA and an FDA-approved product matters. In particular, the FDA's grant of EUA requires little, if any, demonstration that the EUA product is safe and effective. Nor does the EUA include FDA review or approval of manufacturing processes, facilities, storage, distribution, or quality control procedures. This is why the FDA has acknowledged the products are "legally distinct.'' [MORE] and [MORE]
An amicus brief filed by Defending The Republic (DTR) in NFIB v. OSHA importantly pointed out the following;
Important Differences Between EUA and FDA-Approved Vaccines
“There are significant differences between the FDA's approval standards and the EUA standards. EUA vaccines require little to no proof of safety or efficacy. FDA vaccine approvals do.
The FDA may grant an EUA where: (1) the HHS Secretary has declared a public health emergency that justifies the use of an EUA, see 21 U.S.C. § 360bbb- 3(b)(1); and (2) the FDA finds that "there is no adequate, approved, and available alternative to the product for diagnosing, preventing, or treating" the disease in question. 21 U.S.C. § 360bbb-3(c)(3).
The differences between licensed vaccines and those subject to an EUA render them "legally distinct." First, the requirements for efficacy are much lower for EUA products than for licensed products. EUAs require only a showing that, based on scientific evidence "if available," "it is reasonable to believe," the product "may be effective" in treating or preventing the disease. 21 U.S.C. §360bbb-3(c)(2)(A).
Second, the safety requirements are minimal, requiring only that the FDA conclude that the "known and potential benefits ... outweigh the known and potential risks" of the product, considering the risks of the disease. 21 U.S.C. §360bbb-3(c)(2)(B). There is no requirement that the FDA know the potential risks of the product.
In comparison, vaccines that go through traditional FDA review typically take 10 years or more to reach approval. And the approval process compiles more information on the risks of the vaccine, gathered through lab testing and clinical trials, "to assess the safety and effectiveness of each vaccine.''
The Right to Refuse an EUA Vaccine
The FDA's grant of an EUA is subject to informed consent requirements to "ensure that individuals to whom the product is administered are informed" that they have "the option to accept or refuse administration of the product." 21 U.S.C. § 360bbb- 3(e)(1)(A)(ii)(III).
For the three COVID-19 vaccines, FDA implemented the "option to accept or refuse" condition described in Section 564(e)(1)(A)(ii)(III) in each letter granting the EUA by requiring that FDA's "Fact Sheet for Recipients and Caregivers" be made available to every potential vaccine recipient. These include the statement that the recipient "has the option to accept or refuse" the vaccine. Moreover, the EUA label itself must expressly state that the recipient has a "right to refuse" administration of the EUA product.
Informed Consent Rights
The norm of informed consent has been "firmly embedded" in U.S. law and FDA regulations for nearly 60 years. Adullahi v. Pfizer, Inc., 562 F.3d 163, 182 (2d Cir. 2009). Congress first enacted this requirement in 1962 drawing on the Nuremberg Code and the Helsinki Declaration, "which suggests the government conceived of these sources' articulation of the norm as a binding legal obligation." Adullahi, 562 F.3d at 182. Informed consent requirements are a cornerstone of FDA rules governing human medical experimentation. See, e.g., 21 C.F.R. §§ 50.20, 50.23-.25, 50.27, 312.20, 312.120 (2008); 45 C.F.R. §§ 46.111, 46.116-117.
EUA and FDA Licensed Products do not have the "Same Formulation" and are not "Interchangeable"
The EUA and licensed versions of Pfizer- BioNTech do not have the "same formulation" as revealed by a simple inspection of the Pfizer Vaccine EUA letters and the Summary Basis for Regulatory Action (SBRA) for Comirnaty. Thus, they cannot be treated as "interchangeable," because there is no legal basis to administer an EUA product as if it were the FDA-licensed product. By definition, they are different.
There is no evidence in the public record for finding that the EUA Pfizer-BioNTech vaccine and FDA-licensed Comirnaty have the "same formulation." There is, however, ample evidence for finding that they do not. The most detailed information on Comirnaty's composition, manufacturing process, manufacturing locations and other matters approved by the FDA is included in the FDA Comirnaty SBRA, nearly all of which is redacted, while most of this information was never made available in the Pfizer- BioNTech EUA applications or authorizations. To the extent such information is available, it reveals differences in the composition of the EUA and the licensed product. There is also no dispute that the FDA EUA does not address manufacturing processes or locations, which are addressed in the Comirnaty license.
For the same reasons, the public record does not support any argument that the two admittedly "legally distinct" products are "interchangeable." "Interchangeable" and "interchangeability" are specifically defined terms in Section 351 of the Public Health Service Act ("PHS Act"), 42 U.S.C. § 262, in relation to a "reference product," which is a biological product licensed under Section 351(a) of the PHS Act, 42 U.S.C. § 262(a). For the purposes of determining "interchangeability," the "reference product" must be an FDA-licensed product; in this case, the FDA- licensed Comirnaty Vaccine. But the "interchangeable" product, the EUA BioNTech Vaccine, must be the subject of a later filed "abbreviated" application under 42 U.S.C. § 262(k), and there is no indication that any such application was ever filed by BioNTech, much less reviewed or approved by the FDA.
Any "interchangeability" determination would therefore reverse the temporal order of the COVID-19 licensed product and the interchangeable product. The reference product under 42 U.S.C. § 262(a) is the first licensed product, and therefore the basis for determining the interchangeability of the later product (i.e., the generic or EUA product). Here, however, the EUA Pfizer-BioNTech Vaccine is the earlier product, while the licensed Comirnaty is the latter product; the earlier EUA product cannot rely on the FDA's safety and efficacy determinations for Comirnaty. Thus, an "interchangeability" determination would be a transparent attempt to retroactively license the earlier EUA Pfizer-BioNTech Vaccine, solely for the purpose of enabling the unlawful vaccine mandate.
Moreover, "FDA licensure does not retroactively apply to vials shipped before [FDA] approval." Austin, 2021 WL 5816632, at *6. Any EUA-labeled vaccines manufactured before licensure and "vaccines produced after August 23 in unapproved facilities--remain 'product[s] authorized for emergency use,"' i.e., EUA rather than licensed products. Id. In any case, such a post hoc interchangeability determination should not even be considered by the Court. "An agency must defend its actions based on the reasons it gave when it acted." DHS v. Regents of the Univ. of Cal., 140 S.Ct. 1891, 1909 (2020). [MORE]
At any rate COVID injections are not actually vaccines because they do not create immunity. The injections are treatments. As such, individuals have a right to refuse medical treatment.
Prosecute Now explains, The uncontroverted medical consensus is that existing Covid-19 injections do not prevent infection or transmission of the coronavirus; i.e., they do not create immunity in the recipients. This is admitted openly today, including by U.S. Health Agencies, which is why the CDC Director stated on CNN, "What the vaccines can't do anymore is prevent transmission.'
The CDC has acknowledged that the “vaccinated” and “unvaccinated” are equally likely to spread the virus.
The Injections do not confer immunity but are claimed to reduce the severity of symptoms experienced by those infected by SARS-CoV-2. They are, therefore, treatments and not vaccines as that term has always been defined in the law. [MORE]
in August of 2021, the CDC changed the definition of "vaccination" from "the act of introducing a vaccine into the body to produce immunity to a specific disease" to "the act of introducing a vaccine into the body to produce protection to a specific disease.’'
However, this newly created CDC definition conflicts with the statutory criteria for a vaccine, which focuses solely upon immunity. In 1986, Congress passed 42 U.S.C. § 300aa-1, which established "a National Vaccine Program to achieve optimal prevention of human infectious diseases through immunization " (emphasis added). Clearly, from both a public health standpoint as well as from a legal standpoint, immunization is the intended sine qua non of vaccination.
That is, the CDC eliminated the word “immunity” from its definitions of “Vaccine” and “Vaccination.” The CDC apparently did so because it recognizes that the Injections do not produce immunity to the disease known as COVID-19. Since they do not create immunity, but are claimed to merely reduce the symptoms of the disease, the so called Covid-19 vaccines are treatments, not vaccines.
Even the FDA has classified them as "CBER-Regulated Biologics" otherwise known as "therapeutics" which fall under the "Coronavirus Treatment Acceleration Program.’'
The medical community, the relevant agencies, and both Pfizer and Moderna -- the manufacturers of the dominant injections -- recognize that the so-called vaccines are therapeutics, or medical treatments. Since they do not achieve immunization, this conclusion is also consistent with Congress' definition of vaccines in establishing the National Vaccine Program in 1986: the "prevention of human infectious diseases through immunization.''
This is a critical factual and legal distinction. The Supreme Court has long held that the right to refuse medical treatment is a fundamental human right. Since the Injections do not stop the transmission of SARS-CoV-2 as a matter of fact, they are not “vaccines” as a matter of law. Instead, they are a therapeutic or medical treatment which individuals have a fundamental human right to refuse. [MORE]
As explained by Dr. Devan Griner’s complaint against the CMS mandate,
“Because the Injections are treatments, and not vaccines, strict scrutiny applies. The US Supreme Court has recognized a “general liberty interest in refusing medical treatment.” Cruzan v. Dir., Mo. Dep’t of Health, 497 U.S. 261, 278, 110 S. Ct. 2841, 2851, 111 L.Ed.2d 224, 242 (1990). It has also recognized that the forcible injection of medication into a nonconsenting person’s body represents a substantial interference with that person’s liberty. Washington v. Harper, 494 U.S. 210, 229, 110 S. Ct. 1028, 1041, 108 L.Ed.2d 178, 203 (1990), see also id. at 223 (further acknowledging in dicta that, outside of the prison context, the right to refuse treatment would be a “fundamental right” subject to strict scrutiny).
It further explained,
As mandated medical treatments are a substantial burden, Defendants must prove that the CMS Mandate is narrowly tailored to meet a compelling interest.
No such compelling interest exists because, as alleged above, the Injections are not effective against the now dominant Omicron variant of SARS-CoV-2 in that they do not prevent the recipient from becoming infected, getting reinfected, or transmitting SARS-CoV-2 to others. Indeed, evidence shows that vaccinated individuals have more SARS-CoV-2 in their nasal passages than unvaccinated people do.
The Injections may have been somewhat effective against the original SARS-CoV- 2 strain, but that strain has come and gone, and the Injections—designed to fight yesterday’s threat—are simply ineffective against the current variant.
Since the Injections are ineffective against the Delta and Omicron viral variants, and the original variant has been supplanted, there can be no compelling interest to mandate their use at this time.”
But even if there were a compelling interest in mandating the Injections, the CMS Mandate is not narrowly tailored to achieve such an interest.
The blanket mandate ignores individual factors increasing or decreasing the risks that the plaintiff—indeed, all healthcare workers—pose to themselves or to others.
Defendants entirely disregard whether employees have already obtained natural immunity despite the fact that natural immunity does actually provide immunity whereas the Injections do not.
Treating all employees the same, regardless of their individual medical status, risk factors, and natural immunity status is not narrowly tailored.
Moreover, the CMS Mandate fails entirely to consider other existing treatment options beyond the Injections as part of a more narrowly tailored approach. 97. Given these facts, as more fully set forth above, the CMS Mandate has no real or substantial relation to public health or is beyond all question, a plain, palpable invasion of rights secured by the fundamental law. Alternatively, the CMS Mandate has no real or substantial relation to public health or is beyond all question, a plain, palpable invasion of rights secured by the fundamental law as to Plaintiff, who already has natural immunity.” [MORE]