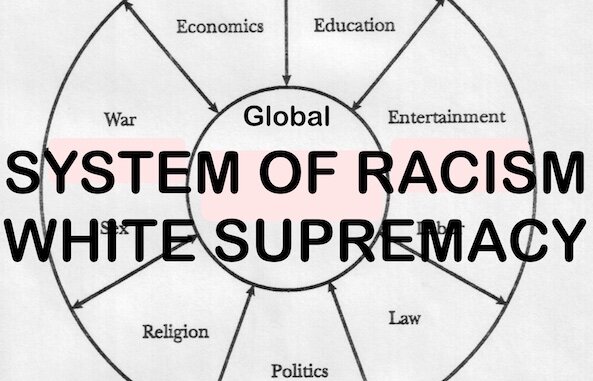
There Are No Licensed COVID Vaccines for Kids Under 12 — But CDC Wants Babies to Get 3 Pfizer Shots by Age 9 Months
/From [HERE] Nine-month-old babies must receive multiple doses of an unlicensed mRNA COVID-19 vaccine to be considered “up to date” with their COVID-19 vaccination, according to the Centers for Disease Control and Prevention (CDC).
The CDC’s updated guidance, issued Aug. 30, states that children — as young as 6 months old — should get either two doses of the 2024-2025 Moderna vaccine or three doses of the 2024-2025 Pfizer-BioNTech vaccine.
If getting the new Pfizer shot, the baby is supposed to receive the first dose at 6 months, the second dose three weeks later and the third dose at least eight weeks after the second dose — meaning, that by 9 months old, babies are supposed to have received three Pfizer shots.
If getting the latest Moderna shot, the CDC recommends babies get the first dose at age 6 months and the second dose a month later.
The latest Pfizer and Moderna COVID-19 shots for children under 12 are unlicensed in the U.S. The U.S. Food and Drug Administration (FDA) has granted only emergency use authorization (EUA) for the vaccines.
Children’s Health Defense (CHD) CEO Mary Holland told The Defender, “The earlier COVID shots have been proven unsafe and ineffective. Now we’re asked to believe that newer versions are miraculously safe and effective?”
“This is an insult to people’s intelligence,” she said, “I pray that parents will have the good sense to say no to these dangerous and unnecessary shots for babies.”
As of July 28, 37,814 deaths following COVID-19 vaccination had been reported to VAERS, the Vaccine Adverse Event Reporting System, run by the FDA and CDC.
Of those, 187 reports were for children and teens under 18. Nearly 13,000 reports listed the age as “unknown.”
VAERS analyst and expert Albert Benavides recently told The Defender he believes VAERS is “throttling” and underreporting deaths of all ages following COVID-19 vaccination.
Meanwhile, the CDC continues to tell the public that COVID-19 vaccines are “safe and effective.”
There’s no licensed COVID vaccine for kids under 12
There are still no licensed COVID-19 vaccines available for children under 12, Hooker said — so all COVID-19 vaccines given to young kids are EUA products.
The FDA’s website on EUA for medical products states that EUA vaccines only have to meet the standard of “may be effective” as long as if, “based on the totality of the scientific evidence, it is reasonable to believe that the product may be effective for the specified use.”
“The ‘may be effective’ standard for EUAs provides for a lower level of evidence than the ‘effectiveness’ standard that FDA uses for product approvals,” the website states.
Before a vaccine can be fully licensed, the vaccine maker typically is required to conduct numerous clinical trials to demonstrate that the product is safe. However, the safety requirements for EUA are more flexible. [MORE]