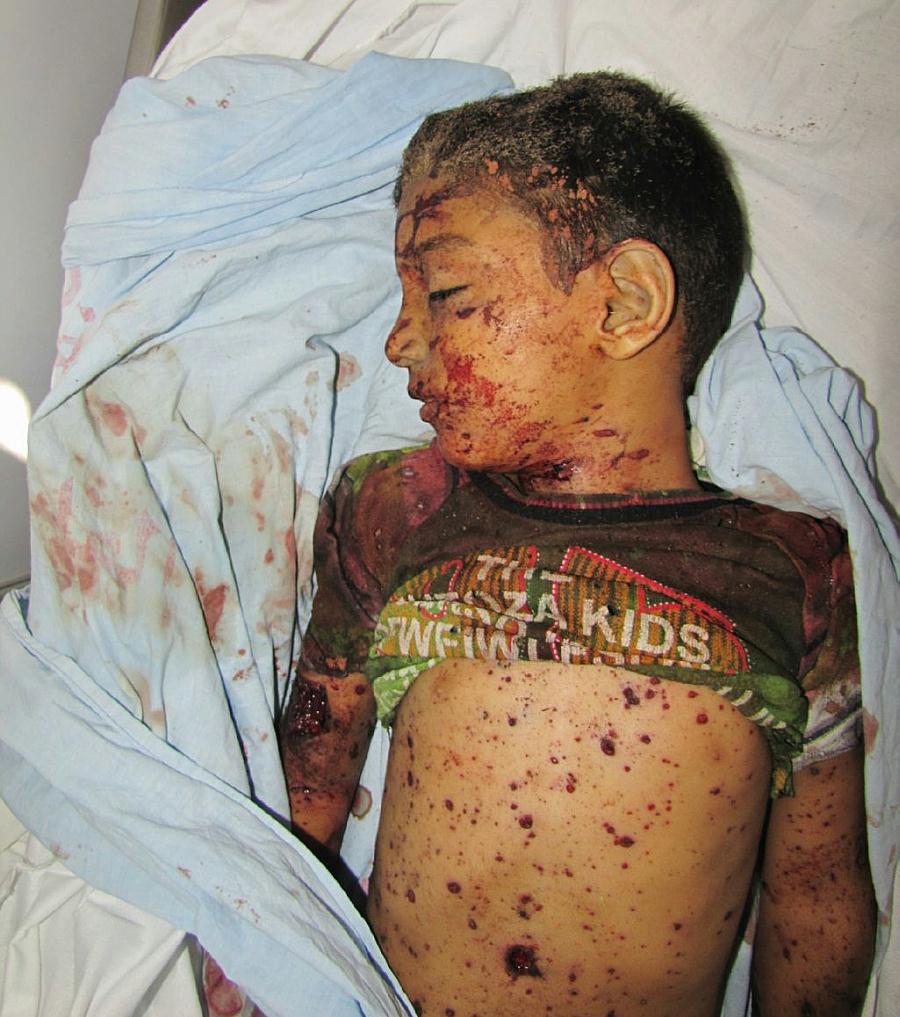
Watts waited for a vaccine he thought was ‘safe and effective’
Watts was a student at Corning Community College in Corning, New York, when in the summer of 2021, the school mandated the COVID-19 vaccine for all students attending fall classes. The mandate was part of the mandate at the State University of New York (SUNY), a network of 64 colleges and universities.
Watts waited to get vaccinated until the FDA “approved” the Pfizer Comirnaty vaccine and got his first dose at Guthrie Robert Packer Hospital in Pennsylvania on Aug. 27, 2021. He was administered the EUA Pfizer BioNTech COVID-19 vaccine.
The FDA approved the Pfizer Comirnaty vaccine on Aug. 23, 2021, but the DOD didn’t make it available.
Despite experiencing side effects from the first dose, Watts understood the vaccine to be “safe and effective,” so he took a second dose at the same location on Sept. 17, 2021.
Following the second dose, Watts experienced more severe side effects, including numbness in his extremities, difficulty grasping and holding objects, a sinus infection, cough and sensitivity to light. He visited the ER at the Guthrie hospital on Oct. 12, 2021, also complaining of a lump on the left side of his neck.
The hospital diagnosed him with sinusitis and prescribed an antibiotic. Watts returned to the ER on October 19, 2021, concerned that he was not improving.
After that, his health continued to decline.
On Oct. 27, 2021, at home with his mother, Watts began coughing up blood and then became unresponsive. His mother called 911 and administered CPR.
Watts was taken to the ER where he was found to be in cardiac arrest and subsequently died. He had no previous medical history that could explain his sudden death. Watts also tested negative for COVID-19 in a post-mortem test.
The medical examiner ruled his cause of death to be “complications of COVID-19 vaccine-related myocarditis.” His death certificate also listed COVID-19 vaccine-related myocarditis as the sole immediate cause of death.
An independent physician, Dr. Sanjay Verma, also attested the vaccine was the proximate cause of death as alleged in the complaint.
PREP Act protects vaccine producers, not vaccine-injured people
Watts’ family first sought compensation for his death under the Health Resources & Services Administration’s Countermeasures Injury Compensation Program (CICP).
The CICP was established under the Public Readiness and Emergency Preparedness (PREP) Act, which protects “covered persons” — such as pharmaceutical companies, or the DOD in this case — from liability for injuries sustained from “countermeasures,” such as vaccines and medications, administered during a public health emergency.
The only exception to PREP Act immunity is if a countermeasure-related injury is caused by “willful misconduct” by a covered person or entity.
Since the start of the pandemic, people claiming injuries related to COVID-19 vaccines and other countermeasures submitted 11,686 requests for compensation.
Of those, only 23 have been declared eligible for compensation. Most of those are undergoing a “medical benefits review” to determine payment. Since last month, when the CICP started making payments to COVID-19 vaccine-injured people, it has made four payments — amounting to a total of $8,592.52. Three of the claims were for myocarditis.
Watts’ family filed a request for benefits with the CICP in August 2022. They received no determination from the CICP within the 240-day period in which the CICP is supposed to respond to complaints.
As a result, to seek compensation for the loss of Watts’ life, his family is suing the DOD.
The DOD, Operation Warp Speed and the COVID vaccines
In January 2020, then-Health Secretary Alex M. Azar of the U.S. Department of Health and Human Services declared a public health emergency for COVID-19.
The emergency declaration allowed the health secretary to make a PREP Act declaration so the FDA could issue an EUA for an unapproved vaccine or other “countermeasure” to address the emergency if the following emergency circumstances exist:
“(1) the existence of a serious or life-threatening disease; (2) a product ‘may be effective’ in treating or preventing it; (3) there is ‘no adequate, approved, and available alternative to the product for diagnosing, preventing or treating such disease or condition;’ (4) a risk-benefit analysis that measures both the known and potential benefits of the product against the known and potential risks of the product is positive; and (5) that the patient’s option to accept or decline the product is protected through informed consent.”
On May 15, 2020, the Trump White House announced Operation Warp Speed — a partnership between the White House and the DOD to accelerate the development, production and distribution of a COVID-19 vaccine.
Two months later, the DOD signed a contract with Pfizer to manufacture hundreds of millions of doses of its mRNA COVID-19 vaccine, guaranteeing that any vaccine produced under the contract would be protected under the PREP Act and therefore not subject to liability.
The FDA issued an EUA for the Pfizer-BioNTech COVID-19 vaccine on Dec. 11, 2020, and Army Gen. Gustave F. Perna, Operation Warp Speed chief operating officer, announced the vaccine would be rapidly distributed across the country.
Drugs fully approved by the FDA must be found to be “safe, pure, and potent,” but EUA drugs are held to a lower standard — they are required only to demonstrate that they “may be effective,” according to the FDA.
But Perna and his boss, Austin III, conveyed the message that the EUA vaccines were “safe and effective,” and urged the healthcare community to do the same, in order to “counter widespread misinformation” about the vaccines, the lawsuit alleges.
After the FDA approved the Comirnaty vaccine, the DOD did not initiate its production and distribution but instead continued to distribute existing Pfizer EUA products.
As a result, although Watts waited for the COVID-19 vaccine to be FDA-approved, he still received a version of the vaccine that had not been FDA-approved as “safe and effective.”
According to the lawsuit, the DOD blurred the line between the two legally distinct vaccines, promoting the idea that the COVID-19 vaccine was FDA-approved and therefore “safe and effective” — while administering the vaccine that was only “authorized,” and therefore not legally allowed to be described as “safe.”
The DOD knowingly blurred this line, the lawsuit alleges, because it had already been found liable for violating informed consent and of imposing an experimental vaccine. In the 2004 case of Doe v. Rumsfeld, et al., a federal court ruled the DOD could not mandate the EUA anthrax vaccine for service members because forcing them to take an experimental vaccine violated their right to informed consent.
That ruling stated that absent informed consent or a presidential waiver, “The United States cannot demand that members of the armed forces also serve as guinea pigs for experimental drugs.”
The current lawsuit further alleges that the DOD knowingly deceived Watts and other Americans for the purpose of mass human experimentation, which violates protections provided by the Nuremberg Code.
According to the complaint, the DOD committed “willful misconduct,” having “deliberately misled Mr. Watts and the public at large by blurring the critical distinction between EUA and fully licensed vaccines,” which would nullify the protections afforded the DOD under the PREP Act.
It concludes that Watts died because he believed he was receiving safe and effective vaccines, but in fact “received the deadly ones.”
The lawsuit seeks “general, special, compensatory and punitive damages.”
Commenting on the significance of the case, Kim Mack Rosenberg, acting outside general counsel for CHD, told The Defender:
“The PREP Act purports to provide an extraordinary liability shield to the government, manufacturers, distributors, and others, related to COVID-19 vaccines and other so-called countermeasures covered by the act. The Watts complaint is an important and unprecedented challenge to that liability shield.
“The complaint threads the act’s needle by pointing the finger squarely at Operation Warp Speed leadership while raising critical legal challenges to the act’s protection, particularly where, as is alleged in the Watts complaint, a defendant like the Department of Defense has engaged in willful misconduct.
“But the complaint does more than that. It will educate about the PREP Act’s far reach, actions by the DOD during the ‘state of emergency,’ and the general lack of accountability for entities and individuals protected by the PREP Act.
“The public needs to understand that this act intentionally allows potentially bad actors to go unpunished. Here, a young man lost his life, and the government has remained silent, hiding behind a legal shield.
“That is not justice for George Watts or anyone else.”